Sandra van Vliet More on research
Cell surface glycosylation is highly diverse, yet it is still unclear how glycan heterogeneity is established and how this impacts tumor-immune cell recognition. This is the main theme of my research.
Outstanding questions that I address within my research are:
1. How are key glycan signatures regulated in cancer cells?
We previously established that an oncogenic mutation in BRAF (BRAFV600E) had a direct impact on the tumor glycome in colon cancer (Lenos Oncotarget 2015) We are currently assessing whether this phenomenon is specific for colon cancer or also occurs in other types of epithelial cancers such as non-small cell lung cancer. Therefore, we profile primary human tumor samples for their surface glycosylation by flow cytometry, immunohistochemistry and mass spectrometry and correlate these to the presence of oncogenes.
2. Are tumor-associated glycan signatures related to anti-tumor immunity?
The presence of elevated levels of immunosuppressive glycans on tumors suggest that glycan-binding receptors actively dampen immune responses directed against the tumor. In human tumor samples we study this by correlating lectin binding to disease progression and immune cell infiltration. In addition, we have generated syngeneic tumor glycovariant cell lines to study the impact of glycosylation on anti-tumor immunity.
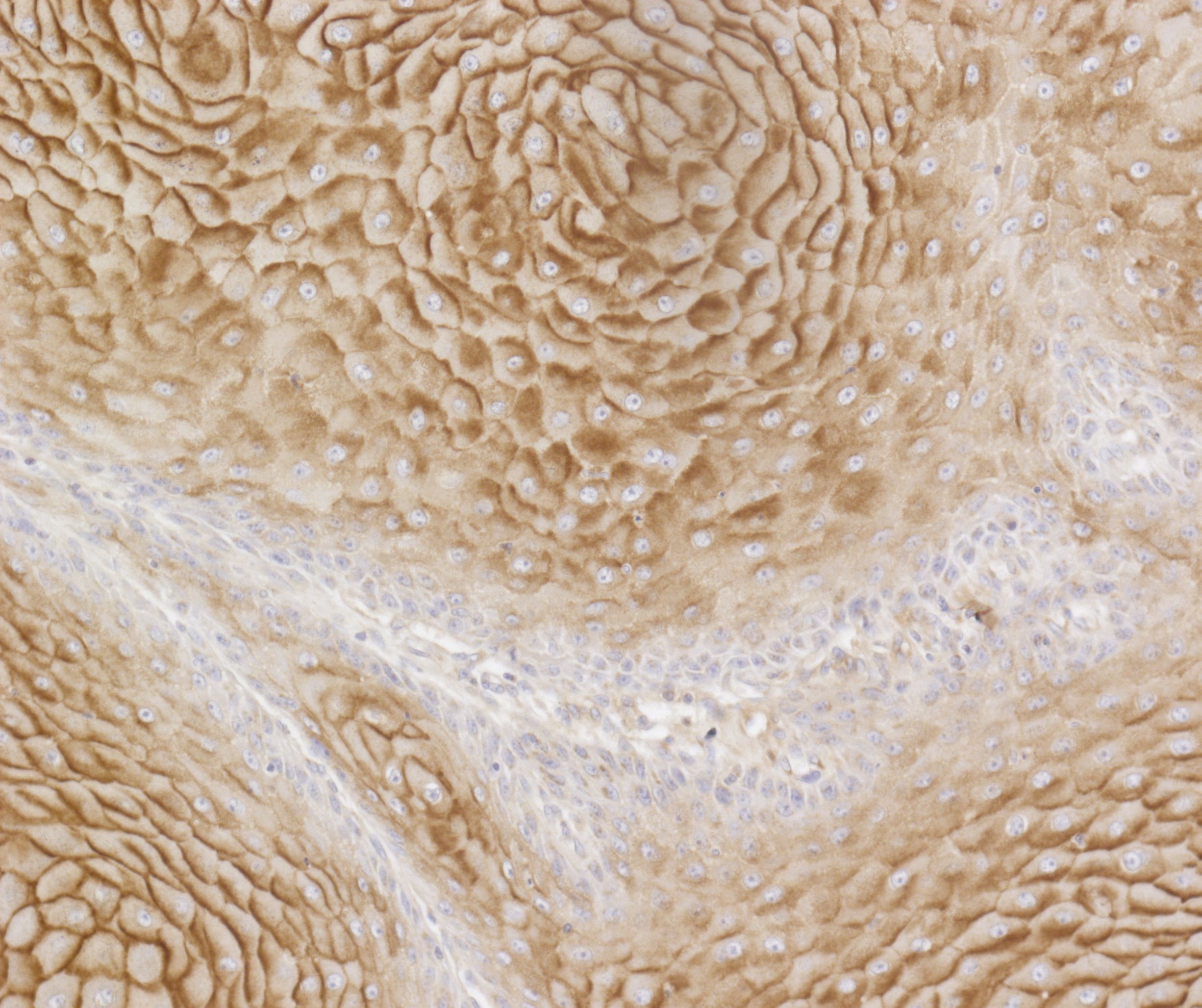
3. Is antigen presenting cell functionality affected by triggering glycan-binding receptors?
To assess the immunological consequences of altered tumor glycosylation on a molecular level, we stimulate glycan-binding receptors on dendritic cells and macrophages with synthetic glycoconjugates. The different cell populations are subsequently analyzed for maturation marker expression, cytokine secretion and their ability to instruct effector and regulatory T cell responses.